Azetukalner (BPD)
Azetukalner is a novel, potent Kv7 potassium channel opener being developed for the treatment of epilepsy, major depressive disorder, and potentially other neurological disorders.
Azetukalner for Bipolar Depression (BPD)
X-CEED, the first of two planned Phase 3 clinical trials evaluating azetukalner in patients with BPD has been initiated to support indication expansion of azetukalner in neuropsychiatry.
About Azetukalner Phase 3 BPD Program
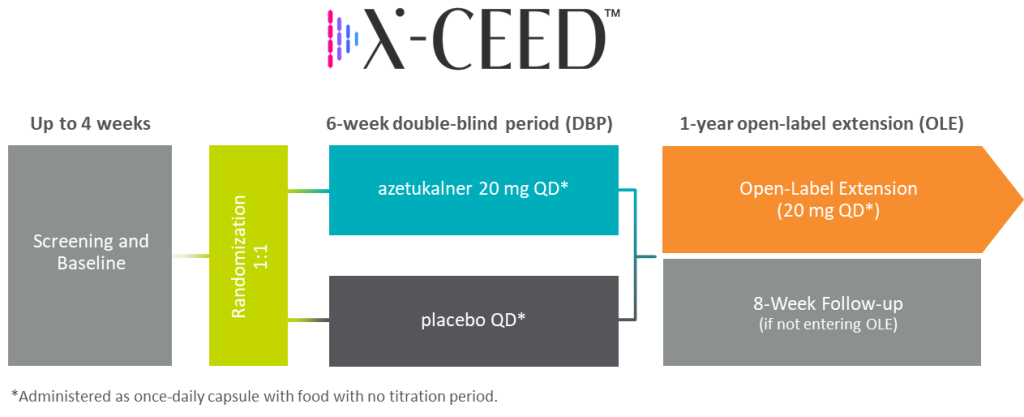
Xenon’s Phase 3 BPD program includes two multicenter, randomized, double-blind, placebo-controlled clinical trials to evaluate the clinical efficacy, safety, and tolerability of 20 mg of azetukalner administered orally with food over the 6-week double-blind period (DBP) as monotherapy treatment in approximately 400 patients per study with bipolar I or II depression (BPD).
Primary efficacy endpoint

The primary efficacy endpoint is the change from baseline in the MADRS score at week 6 in patients who received azetukalner compared to placebo. Upon completion of the DBP, eligible patients may enter an OLE study for up to 12 months.